Factor says everyone knows most drugs fail to get through clinical trials
Health & Biotech
Health & Biotech
Would-be drug developer Factor Therapeutics says it’s well known that 70 per cent of drugs fail in phase 2 clinical trials, and now their disappointed shareholders know too.
The company has been so busy answering questions since its failed wound care clinical trial that it has released an FAQ, in which it says phase 2 clinical trials are “the highest risk”.
Factor (ASX:FTT) was nearly entirely wiped out last week when it revealed that its candidate for treating venous leg ulcers, VF001, failed to show any clinically meaningfully difference on standard care treatment.
The company lost 97 per cent of its value when the market opened after the results were released, and has been trading between 0.2 and 0.4 of a cent ever since.
Everybody knows…
In defending itself, Factor reminded its shareholders after the fact that “it is well recognised” that success rates for moving drugs from phase 2 to 3 are about 30 per cent.
Clinical trials are generally divided into three phases. Phase 1 focuses on safety, phase 2 tests for effectiveness and phase 3 examines whether the new drug is an improvement on existing treatment. Sometimes trials are further divided into parts A and B, where a B stage is generally more rigorous.
Between 2005 and 2016, only 9.6 per cent of drugs made it from phase 1 to market, according to Biotechnology Innovation Organisation data.
Some 63 per cent of drugs make it from phase 1 to phase 2, but only 30.7 per cent of drugs make it out of phase 2.
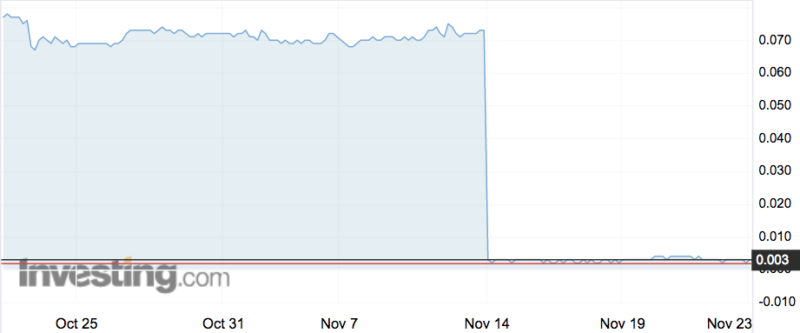
Big disappointment
Many shareholders have been seeking answers as to why VF001 failed at the second phase after promising signs at the first phase.
“Can someone please tell me and everyone else how results from previous studies were so positive, including graphs and photos of wounds healing while the result of this trial was not better than placebo???????” asked one punter on trading forum HotCopper.
“With TIS [Tissue Therapies, the company’s previous name] we were wIth ‘a great product unable to enter in the EU due to a badly run small sized trial’ to now ‘a failure not divergent from a placebo.’ There is something really wrong here. Simply does not make any sense that a good product transforms itself to this,” said another.
But others pointed out the inherent risk of investment in biotechnology companies.
“Ladies and gentlemen, please take this result as actually the absolute norm in the biotech sector. If you invest in biotechs you need to have the attitude that it will most likely fail. All drugs usually go through the same steps and trials to get to commercialisation which test for safety and whether the drug meets its endpoints. The biotech sector is the closest form of gambling on the stockmarket,” said one.