Bionomics says its ‘failed’ PTSD drug might work after all, shares fly 75pc
Health & Biotech
Health & Biotech
Bionomics, one of a number of biotechs decimated by negative clinical trial results last year, says its apparently failed PTSD drug might work after all.
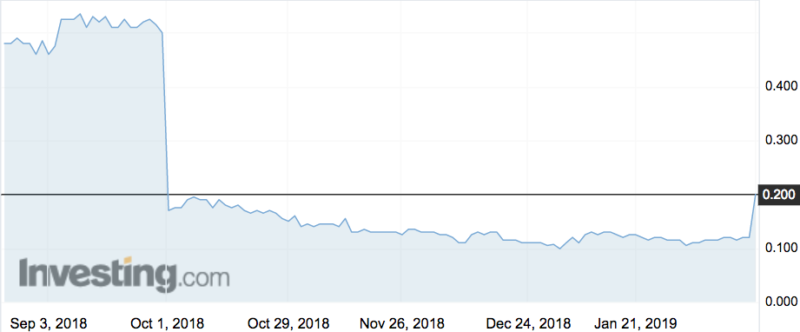
Shares in Bionomics (ASX:BNO) flew 75 per cent this morning to a high of 21c, its highest point since early October.
The drug BNC210 was supposed to improve the quality of life for patients with post traumatic stress disorder, but in October the company said the trial failed to show clear beneficial effects on improving PTSD symptoms.
BNO shares lost 68 per cent of their value that day.
But now the company says further analysis has revealed the drug does have an impact after all.
Bionomics conducted exposure-response analysis, which analyses levels of the drug in a patient’s bloodstream regardless of the administered dose, to relate drug exposure to the response measured by patients assessed under the CAPS-5 (clinically administered PTSD scale) system, a 30-item questionnaire used by clinicians to assess symptoms.
A spokesman for the company said the original trial included people who didn’t take the drug properly — with food — and later analysis showed that among those who did take the drug correctly, it remained in the patients’ bloodstream and did have a positive effect on PTSD symptoms.
Bionomics chairman Dr Errol De Souza says it vindicates the company’s decision to seek to treat PTSD with BNC210, even though it stopped nearly all work on the drug, lost its CEO and underwent a recapitalisation and strategic review after the initial news.
“At the time of the top-line data announcement of 2 October 2018, the results of the complex and time-consuming drug exposure analyses were not available and could not have readily been foreseen,” Dr De Souza told investors.
“The results of the further analysis are meaningful for future development of BNC210 and they support its continued development for PTSD, as well as other indications, and our ongoing partnering activities.”
Bionomics now says it is completely revitalising BNC210 with regards to PTSD, saying it will seek guidance from the US Food and Drug Administration (FDA) on the next steps including the design of a further trial and whether the drug is eligible for fast track designation.
The new data will also be taken into consideration for the strategic review, being conducted in conjunction with investment bank advisors Greenhill.
Professor Mats O Karlsson, who works at Uppsala University, the institution which performed a pharmacometric analysis on the data, said that as long as patients took the drug as instructed, it could work.
“Exposure-response modelling has shown the potential for BNC210 to have significant benefit in PTSD provided that adequate blood levels are achieved,” he said. “This analysis provides a basis for optimal design of future trials to demonstrate efficacy.”
The company is also testing BNC210 in elderly patients with agitation issues and has a range of other candidates in experimental trials.